Charles Law
PURPOSE:
This experiment explores the relationship between volume and temperature of air or some other gas.
PROCEDURE:
- Read the MSDS sheets for safety precautions and disposal of chemicals.
- Place a one hole stoppered 250 ml erlenmeyer flask in a 600 ml boiling water bath. The glass and rubber tubing should extend into a beaker containing 150 ml of water. The level of the water should be near the level of the bottom of the stopper in the flask.
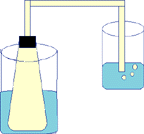
- When the air no longer bubbles from the tubing after boiling, this means the air has reached boiling temperature. Record the temperature of the boiling water.
- After five (5) minutes; carefully replace the boiling water bath with cold tap water. Water from the displacement beaker will be drawn into the flask. Why?
- When the water flow has ceased, adjust the level of the water between the beaker and the flask and then clamp the hose.
- Record the temperature of the water bath.
- Record the volume of water collected.
- Fill the flask with water to location of the bottom of the stopper. Measure and record this volume. The volume of the flask is the total volume of the gas at the higher temperature.
DATA:
- Higher temperature__________
- Lower temperature_______________
- volume of gas at the higher temperature_________
- volume of water after cooling__(-)______________
- experimental volume of gas at low temperatue___________
- theoretical volume of gas at lower temperature_________
- percent error________